Radiopharmaceuticals – the key to future cancer care?

Marika Nestor and her research team have taken a new step in cancer precision medicine with a completely new radiopharmaceutical. Photo: Mikael Wallerstedt.
Precision medicine is often described as the future of healthcare, offering an effective way to create more individualised treatments. Cancer treatment, in particular, is an area where precision medicine may serve a crucial purpose, and one significant form of treatment could be radiopharmaceuticals.
Marika Nestor, Professor in Biomedical Radiation Sciences at the Department of Immunology, Genetics, and Pathology, didn’t initially envision herself researching and working with radiopharmaceuticals and cancer precision medicine. However, with a degree from the molecular biotechnology engineering program, it has proven to be a perfect fit.
“Basically, ever since I was young, I have enjoyed inventing, figuring things out, and solving problems. After completing my thesis project, I knew I wanted to engage in some form of medical research and felt that cancer research was relevant because it is a disease that affects everyone, either directly or through the illness of a loved one,” she explains.
“Early on, I also found the connection between radioactivity and treatment interesting and fascinating, as it encompasses several aspects and elements from what I studied at university.”
Precision medicine – the future of healthcare
This is precisely the area where Marika and her research group focus their efforts. Radiopharmaceuticals, in simple terms, involve using cancer-targeting molecules, such as antibodies, coupled with a “linked” radionuclide to both locate and treat cancer more precisely and accurately. It is essentially what is referred to as precision medicine, a concept that many, including Marika Nestor, see as the future.
“That’s the direction we are moving towards and must move towards, especially for more complex diseases like cancer. Not everyone is cured today, leading to unnecessary treatments in some cases. Moreover, medications are expensive, and therefore, methods like precision medicine are needed to determine each individual patient’s condition from the start, allowing for a more tailored treatment. The process itself may be costly, but the precision achieved will make it worthwhile. Overall, there are numerous benefits,” she asserts.
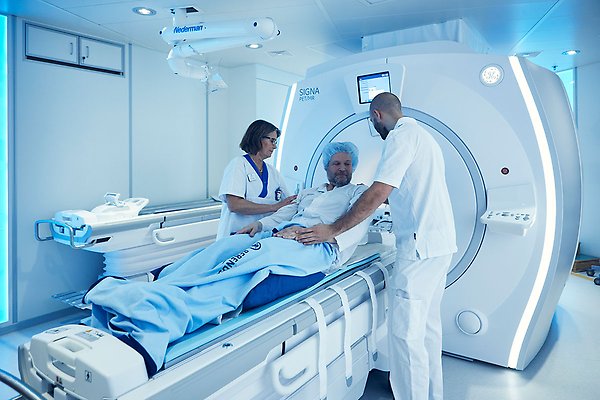
With a PET or SPECT camera, you can see where the molecules administered in the patient have ended up, and whether they have accumulated as intended to a high degree in the tumor compared to the “normal organs”. Photo: Johan Alp.
Examining each patient individually to tailor treatment to the specific cancer type and tumour is what makes radiopharmaceuticals particularly hopeful for the future. It’s not just a therapeutic drug; it can also be used for so-called “imaging”.
“The radiopharmaceuticals we research have the advantage of being used both to initially locate and characterise the tumour and any metastases, and subsequently, for treatment. It depends on the radionuclide attached to the targeting molecule. Some radionuclides emit radiation that can be detected outside the body with PET or SPECT cameras, for example. You can then see where the administered molecules have ended up in the patient and whether they have accumulated as intended to a high degree in the tumour compared to ‘normal organs’,” Marika explains.
“Once it’s confirmed through imaging that the cancer targeting has worked, we can proceed to provide the same patient with treatment using a therapeutic version of the same drug. In this case, we replace the imaging radionuclide with a variant emitting a different type of radiation that is more therapeutic. The molecules attached to the cancer cells in the tumour will then emit radiation. It’s essentially like a focused radiation therapy that occurs inside the body, concentrated specifically to the tumour or tumours.”
But how do the molecules “know” where to go? How do they find their way to the tumour? The answer lies in the surface of the body’s cells.
“It’s not that the molecules with the radionuclide are attracted to the tumour, so to speak. It’s about differences in the surface of normal cells and cancer cells. For example, cancer cells may have much more of certain growth receptors to grow and divide. These can be used as targets, and molecules can be designed to fit and bind to that specific target. The molecule can consist of an antibody designed to find a particular thing. They circulate in the blood until they find their target and attach to it.”
The intensity of the radiation from the radionuclides attached to the molecules depends on the specific radionuclide used.
“Uppsala University is at the forefront”
For her research group, things look promising, and in fact, a drug developed at SciLifeLab in Uppsala is set to undergo clinical trials this year. However, let’s return to that later. First, we can acknowledge that Uppsala University and Uppsala University Hospital are at the forefront in this field and within cancer precision medicine as a whole.
“My perception is that we are at the forefront and have researchers here who are among the best in the world. We are very good at translating things to the clinic, as exemplified by CAR-T cells, which Uppsala was the first in Europe to use in treatment. The mix of basic research and translational research entering clinical trials is excellent. In my field of work with imaging, we are also advanced, with a strong tradition with our PET centre. We have access to fantastic facilities here that create very favourable conditions to be at the forefront in crucial research areas.”
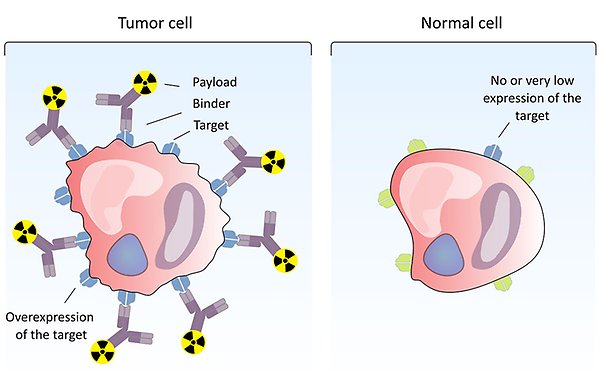
A radiolabelled compound binds to a target that is overexpressed on the surface of tumour cells, while normal cells do not (or to a very small extent) express the target. Thus, the radionuclide will accumulate on tumour cells but not in normal tissue. Illustration: Diana Spiegelberg.
New patent brings hope
Currently, two radioactive drugs are approved for use in Sweden: Lutathera and Pluvicto. The former treats neuroendocrine tumours (NET) and has been shown to extend life. Pluvicto is used to detect prostate cancer. However, more are in development, leading us to the drug Marika has been involved in developing.
“One of the projects we’ve had at SciLifeLab in Uppsala has focused on developing an antibody labelled with a radionuclide for molecular treatment of primarily anaplastic thyroid cancer, but also other forms. It will enter clinical trials this year.”
For this purpose, Marika Nestor has been involved in starting the company Akiram Therapeutics.
“We realised that it was something we wouldn’t be able to manage within our research activities due to the costs involved in clinical trials. So, we obtained a patent for this molecule and established a company instead. It has been a new and exciting journey.”
Why did you choose thyroid cancer?
“It was the cancer type where we believed there was the greatest medical need, especially since there is no cure for anaplastic thyroid cancer today. It is one of the worst cancers you can get, with an average survival of just a few months after diagnosis. When the target I was working on turned out to be present in anaplastic thyroid cancer, it became natural to focus on that cancer type for our work.”
Where all of this will lead is difficult to predict at such an early stage. The only thing to go on is the results from animal trials, where almost all animals have been cured with just one dose.
“But that’s an unfair model and cannot be directly transferred. A human is not a 70-kilo mouse. It will look different. Our trial this year will mostly focus on the pharmacokinetics and safety around the drug. But we also hope, through imaging, to see the drug accumulating in the patient’s tumour and then perhaps be able to administer additional doses to observe a therapeutic effect. Looking at the approved drugs today, they have an effect, but it’s not like everyone is cured. We hope to see very good responses in some cases, while we may see more of a slowing effect in others. Therefore, there is still a need for other efforts that can further enhance the effect of the radiopharmaceutical treatment, and that’s the next step,” concludes Marika Nestor.
Robin Widing
