Peptide Chemical Biology
Description
Our research is conducted at the interface between chemistry and biology. Our ultimate aim is to understand and explore the chemistry of nature and and natural processes that can be useful for drug discovery. In particular we explore the fundamentals of peptides with possible pharmaceutical and medical applications.
Peptides and proteins are produced in all living organisms to fulfill important biological functions. Hence one would think that they would constitute one of the most common types of drug substances. However, the use of peptides and proteins is hampered becasue of their lack of stability
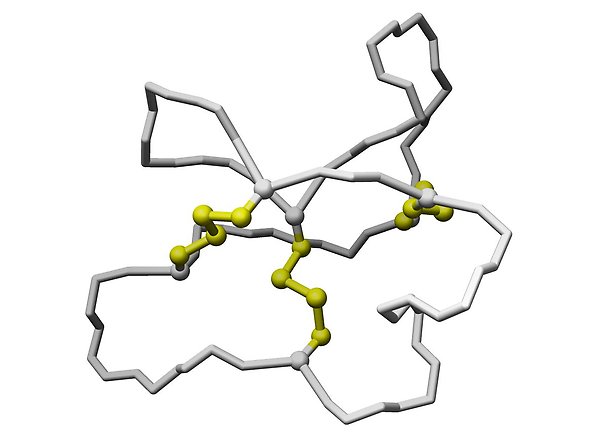
We have found that some plants produce a cocktail of very unusual polypeptides that are head-to-tail-cyclized via amide bonds. These cyclic polypeptides, known as cyclotides (cyclo-peptides), are around 30 amino acid residues long and show an exceptional chemical and biological stability. This is partly explained by their cyclic backbone, but in addition they contain six conserved cysteine residues that are involved in three disulfide bonds. Together these features confine the so called cyclic cystine knot motif (CCK), as shown in the figure below.

Viola odorata, a cyclotide
expressing plant
The central core of the cyclotides consists of three disulfides, marked in yellow and represented in "stick and ball" mode in the figure below. The disulfide bonds to the left and to the right and their connecting backbone (marked in grey) form a ring through which the third disulfide bond is threaded. This is the cystine knot, which together with the cyclic backbone defines the cyclic cystine knot motif.
The cyclic cystine knot makes cyclotides highly suitable for protein engineering applications.
Biologically active peptide sequences can be succesfully be inserted into the stable cyclotide scaffold. Native cyclotides also show a variety of biological activities, such as contraction of the uterus (which was why the first cyclotide kalata B1 was discovered), and anti-HIV and insecticidal activity.
We have recently shown that they also have potent cytotoxic and antibacterial effects, which is currently followed up by structure activity studies and studies of cyclotides effect on artifical cell membranes in the form of liposomes.
For more information, including the possibilities to do student projects in our group, please follow the links to the left or contact Ulf Göransson. You can also find more information about cyclotides in particular in Cybase, a database dedicated to cyclic proteins, and cystine knotted peptides in general in The Knottin database.
Our research is supported by the Swedish Research Council Vetenskapsrådet, and the Swedish Research Council FORMAS.
Research project
HONEYBEE PEPTIDES
Honeybees are the source of many products that have been used in traditional medicine from ancient times. These products have diverse biological activities, and many of them are cytotoxic activity. In this project we focus on the isolation and identification of low abundant bioactive peptides.
ISOLATION AND IDENTIFICATION OF SECONDARY METABOLITES FROM MICROORGANISMS
Multi resistant bacteria as MRSA (Meticillin Resistant Staphylococcus Aureus) and ESBL (Extended Spectrum BetaLactamase) Escherichia coli threaten to cause new epidemics. Our research group combines pharmacognosy with microbiology and focus on samples from extreme microenvironments. We isolate and identify the microorganisms and cultivate them in lab environment. We then observe how they survive MRSA, ESBL, and other multi resistant bacteria and yeast. The microbiology part includes macro-and microscopic identification of microorganisms, and antibiotic assay. The chemical process of structure elucidation of the secondary metabolites include Solid Phase Extraction (SPE), Thin Layer Chromatography (TLC), High Performance Liquid Chromatography (HPLC), Mass Spectrometry (Q-Tof and iontrap MS), and Nuclear Magnetic Resonance Spectroscopy (NMR).
BIOENGINEERING OF THE CYCLOTIDE FRAMEWORK
The aim of this project is to exploit the cyclic cystine knot motif of cyclotides as a scaffold for drug design. This includes introducing novel bioactivities into that stable scaffold and to use cyclotides as vehicles for drug transport. In addition, we are also designing stable cyclic peptides with antibacterial activity satisfying low cytotoxicity and resistance susceptibility, as alternatives for conventional antibiotics. Solid phase peptide synthesis, protein NMR and mass spectrometry are the techniques that are mostly used in our research.
SCREENING CYCLOTIDES WITH SELECTIVE ACTIVITY
The goal is to test the therapeutic potential of extracted/ chemically synthesized cyclotides as anti-cancer and antimicrobial compounds. To date, a suite of different cyclotides have been tested for their cytotoxic activity, and We have assessed the activity of cyclotide variants in vitro, and aim to investigate structure-activity relationships using cytotoxicity as a model activity.
GENOMIC ORGANIZATION OF CYCLOTIDES
Cyclotides have been found within 29 species of plants, each plant usually having an arsenal of many variants differing in only a few residues. There are at present no genomic data available, meaning that their gene organization, expression factors, and evolution are unknown. To facilitate the search for new cyclotides, increase production output, and the understanding of post transcriptional modification mechanisms, including possible recombination, this information is of broad interest for the field.
BIOACTIVE COMPOUNDS FROM EGYPTIAN MEDICINAL PLANTS
Plants have always been a rich source of lead compounds, many of them are useful drugs in themselves and others that have been the basis for synthetic drugs. This project aims at the discovery of new anticancer agents from the medicinal plants of Egypt. A series of plants have been collected, identified and screened against human cancer cells. Most of these plants originate from the traditional by Bedouins in Sinai. On the photo above, Prof Hesham El-Seedi and doctor Ahmed is collecting plants in the mountains near St Catherine.
BIOACTIVE COMPOUNDS FROM SRI LANKA
International collaboration between University of Colombo (PI: Dr. Chamari Hettiarachchi, and in collaboration with Prof. E. Dilip de Silva) and Uppsala University (PI: Dr. Sunithi Gunasekera) supported by the Swedish Research Council's Grant scheme 'Swedish Research Links' (2014-2016). The aim is to characterise the bioactive constituents in Sri Lankan Traditional Ayurvedic Medicinal System.
